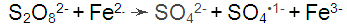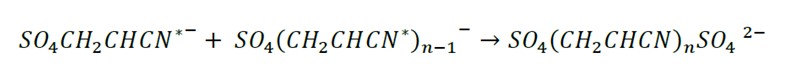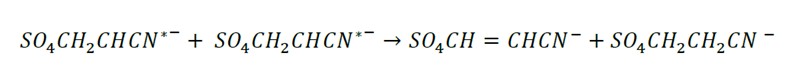SUSPENSION POLYMERIZATION
Home > Suspended Polymerization
Of all known polymerization processes, the main process used in the production of polyacrylonitrile (PAN) on an industrial scale for the manufacture of acrylic fibers is Suspension Polymerization.
In Suspension Polymerization, the most common redox system for acrylonitrile polymerization is persulfate ions, ferric iron and bisulfite based, at pH between 2 to 3.5 where bisulfite ions (HSO
3-) are predominant and ferric ions generated by oxidation are sufficiently soluble. When necessary, a solution of sulfuric acid is added to maintain the pH in that range. The polymerization initiation stage occurs with the formation of the free radical through two reactions: the oxidation of ferrous cations by persulfate and the reduction of ferric cations by bisulfite ion. These reactions produce free radicals of sulfate * and sulfite * that react with the monomer, rapidly starting the growth of the chain. In addition to acting as a reducing agent, the bisulfite ion works as a chain transfer agent that reacts with the radical of the growing polymer, thus controlling the molar mass of the polymer without practically changing the polymerization speed.
The polymerization of acrylonitrile and its transformation into polyacrylonitrile occurs in the following steps using a Redox Persulfate / Bisulfite / Fe
+3/Fe
+2 system:
Initiation: Formation of free radicals by the oxidation of Fe
2+ by the persulfate anion (S
2O
82-) and by the reduction of Fe
3+ by the bisulfite anion (HSO
3-).
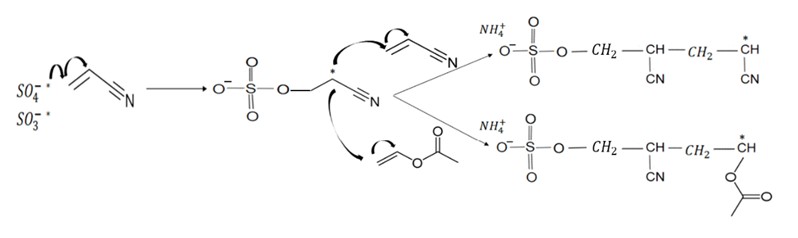
Propagation: The free radicals formed - SO
4- * and SO
3- * - react quickly with the vinyl bonds of acrylonitrile dissolved in water.
Termination: The growth of the polymer chain ends by 3 mechanisms with the sulfur anions entering at the beginning and at the end of the polymer chain.
Radical recombination:
Disproportionation:
As shown in the PAN polymerization process flowchart via redox, the copolymerization by aqueous suspension of acrylonitrile (AN) with a neutral comonomer (such as methyl acrylate or vinyl acetate):
Starting from the tanks (1), the dosage of acrylonitrile (AN), water and comonomer begins in a pre-mix tank (2).
Using pumps specifically programmed with a computer, the mixture of AN, comonomers and demineralized water is carried by means of a centrifugal pump (3) to the polymerization reactor (4) which consists of a vessel made of stainless steel or aluminum alloy , jacketed, and closed at atmospheric pressure.
The reactor (previously heated with steam from a boiler) is about 2/3 of its volume filled with the mixture of demineralized water with the catalyst and activator (5).
Thus, the pre-mix tank solution meets the necessary conditions to start the copolymerization during its launch in the reactor.
The AN copolymerization reaction is highly exothermic and, therefore, the reactor must be cooled by the circulation of water from a chilled water unit (chiller) that generally maintains the reaction temperature constant between 55 to 60 ° C.
The aqueous slurry that forms inside the reactor is continuously agitated by a shaft with propellers at a rotation between 15 and 20 rpm (6) that maintains the suspension viscosity at around 50 cP.
The polymerization continues until the reactor is completely filled and its overflow occurs through a tubular duct (7) coupled between the reactor cover and the unpolymerized monomer recovery column (8). In this duct, the redox reaction that forms free radicals is interrupted by the addition of ethylene-diamine-tetra-acetic acid (EDTA) (9) which acts as a chelating agent, forming stable complexes with iron ions.
The EDTA is constantly injected into the duct towards the unpolymerized monomer recovery column.
Upon falling into the recovery column, the aqueous slurry encounters an upward stream of superheated steam that acts on the volatilization of the monomers that did not react in the reactor, thus starting a process of recovery of these unpolymerized monomers.
The water vapor drags the monomers to a heat exchanger (10) cooled by the circulation of chilled water, recovering the monomers by condensation (11), therefore returning to the pre-mixing tank.
The aqueous slurry, as it passes through the unpolymerized monomer recovery column, can be collected in a reservoir where it is pumped (12) to later steps (13) that will culminate in the dry PAN polymer powder.
A vacuum washing and filtering system removesthe water, dissolved salts and residual EDTA.
Then, the still wet polymer mass is pressed continuously in the form of pellets which provides greater drying efficiency and better storage of the finished polymer in silos with capacity of several tons.
After drying, the PAN is a white powder, insoluble in water and only soluble in highly polar solvents such as dimethylformamide (DMF), dimethylacetamide (DMAc), Dimethylsulfoxide (DMSO), nitric acid, zinc chloride solutions and sodium and ammonium thiocyanates.