POLYACRYLONITRILE PROPERTIES
Home > Polyacrylonitrile Properties
PAN is a polymer with the formula (C
3H
3N)
n that has unique properties.
Homopolymeric Polyacrylonitrile (PAN)
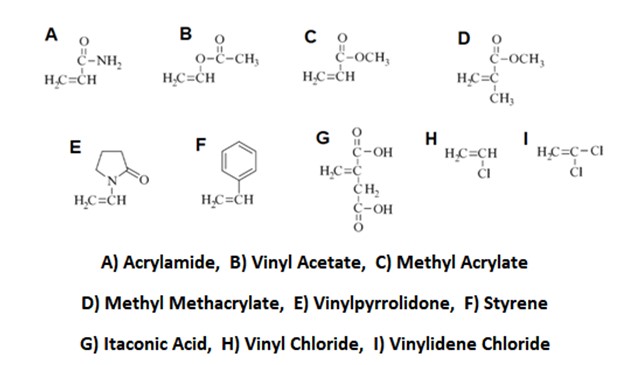
Almost all PAN produced for commercial applications are copolymers obtained from mixing acrylonitrile with other monomers, mainly vinyl esters (vinyl acetate, methyl acrylate and methyl methacrylate) in textile applications. In carbon fiber applications, mainly acrylamide, vinylpyrrolidone and itaconic acid are used. In anti-flame modacrylic fibers, vinyl chloride and vinylidene chloride are used. Styrene is used in SAN thermoplastic resin and in ABS.
Among the characteristics to be highlighted in the PAN:
- It is the most resistant polymer among all to degradation by sunlight, mainly by ultraviolet rays.
- Has the ability to form oriented fibers.
- It is quite inert and resistant to most organic solvents and acids, being only attacked by highly polar liquids and concentrated solutions of bases.
- Its fibers are resistant to breakage, produce high volume, are soft, comfortable, and thermal insulating, having properties similar to natural wool.
- In the form of fibers, when heated, it does not melt and maintains its morphological structure, a property that is used for the production of carbon fiber, insulating fibers, anti-flame fibers and blankets for the filtration of hot gases.
Chemical formula |
|
IUPAC Name |
Polyacrylonitrile |
CAS Number |
25014-41-9 |
Structure |
|
Centesimal composition |
C 67,91%, H 5,7%, N 26,4% |
Appearance |
White Solid |
Density |
1,184 g/cm³ |
Fusion point |
Above 300ºC |
Boiling point |
Degrades |
Glass transition temperature |
Approx. 95ºC |
Solubility in water |
Insoluble |
Main Solvents |
Dimethylformamide, Dimethylacetamide, EthyleneCarbonate, PropyleneCarbonate, SodiumThiocyanate, ZincChloride, NitricAcid |
Thermal Properties of PAN
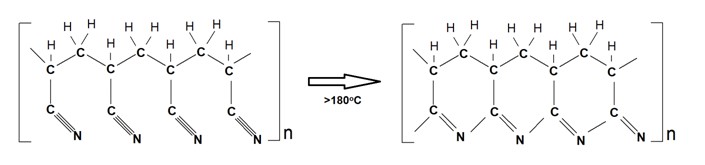
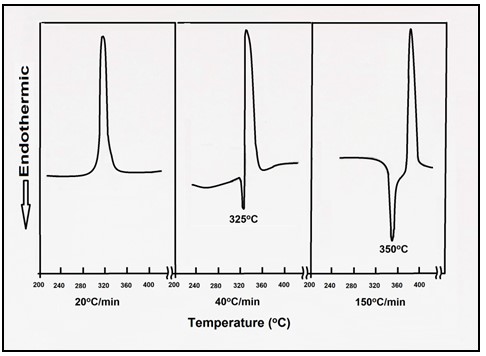
PAN does not melt under normal conditions and degrades before melting. Only by DSC calorimetry can the peak of its melting be observed, above 300ºC, if the heating rate is 30 ºC per minute or more. Heated above 180ºC it turns into a rigid structure with energy release, a phenomenon known as cyclization.
The higher the temperature, the faster the release of energy is, which can cause the polymer to burn.
If the heating is slow and the heat released is removed, the PAN fibers can maintain the fibrillar structure and when heated above 1000ºC, they are transformed into carbon fibers with a content greater than 90% of this element. This property makes PAN the best polymer to be used in the production of carbon fiber.
PAN cyclization reaction
Its color is white, but depending on the temperature, it may acquire a yellowish color that tends to black, due to the cyclization and formation of conjugated bonds in the polymer chain.
Heating of PAN-co-PS fibers in isothermal conditions at 190ºC in air
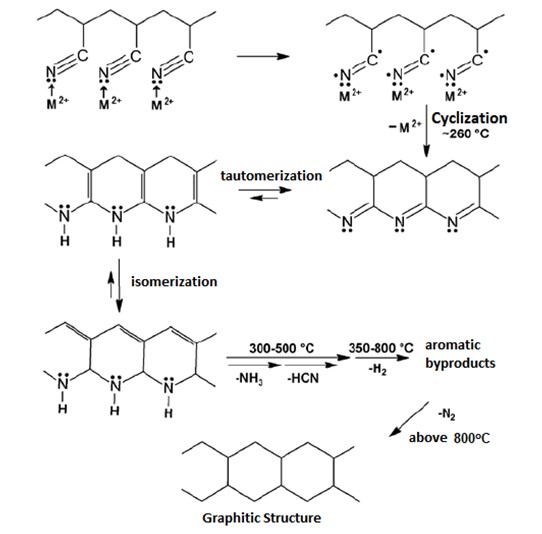
During heating, the PAN structure undergoes several changes, since its oxidation, if heated in the air, and also undergoing mass loss due to the release of different types of gases. Some authors describe the thermal decomposition of PAN with the following stages:
Stage |
Gases Released |
Temperature Range (ºC) |
| I |
Small release of H2O |
Until 250ºC |
| II |
Release of H2O, NH3, and little HCN and CO2 |
300ºC to 450ºC |
| III |
There is an increase in the release of CO2 and HCN, along with H2O and NH3 and hydrocarbons |
450ºC to 600ºC |
| IV |
H2 starts to be released due to cracking of hydrocarbons along with HCN and CO.
Decreased release of NH3, H2O and CO2 that are converted to C, H2, N2, HCN and CO |
600ºC to 900ºC |
PAN thermal degradation model proposed in the article
“Processing and Application of Ceramics” 4 [2] (2010) 59–62
PAN Infrared Spectrum
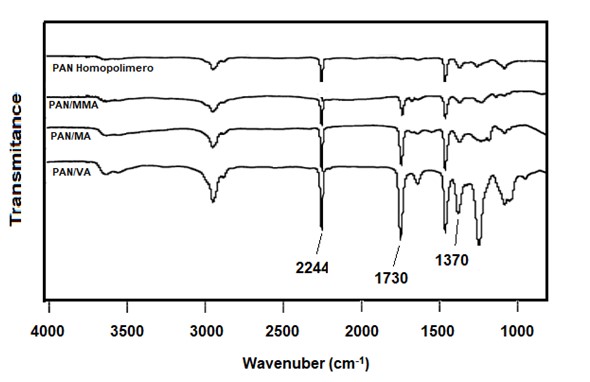
PAN's infrared spectrum easily shows the identity of the polymer. The characteristic peak of PAN and acrylic fibers is located at 2244 cm-1 and is attributed to the strong band of the CN group. The absence of a band at 1730 cm-1 indicates that it is a homopolymer, since no comonomer was used.
When a strong band is observed at 1730 cm-1 for the CO group, it is associated with the presence of a comonomer with an ester group, such as vinyl acetate and methyl acrylate, or carbonyl itaconic acid. In the 1200 cm-1 region, the presence of methyl acrylate is indicated by a peak at 1170 cm-1 associated with other small peaks at 1204, 1229 and 1250 cm-1. A peak at 1240 cm-1 indicates the presence of vinyl acetate as a comonomer. Methyl methacrylate is identified by the bands at 1220 and 1130 cm-1. Acrylamide in PAN can be indicated due to the strong absorption band at 1684 cm-1 and vinylpyrrolidone with a strong band at 1670 cm-1. The comonomer content in PAN of the vinyl ester type can be determined by the ratio of the intensities of the 2244 cm-1 (CN) band to the 1730 cm-1 (CO) carboxyl band, compared with standards previously analyzed by chemical methods.
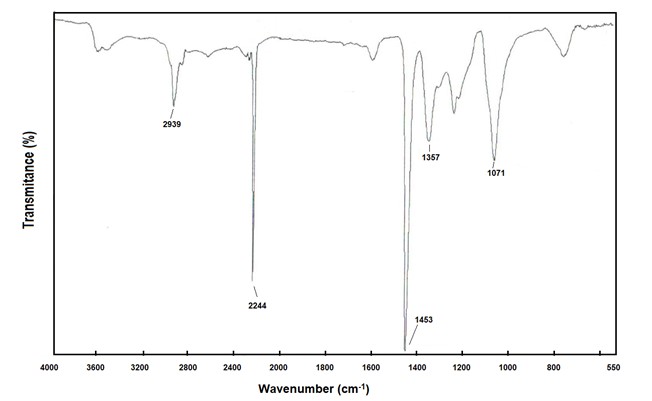
Infrared Spectrum - PAN Homopolymer
Spectral Comparison of several polyacrylonitrile polymers
Pan Homopolymer
PAN-co-Methyl Polymethacrylate (PAN / MMA)
PAN-co-Methyl Polyacrylate (PAN / MA)
PAN-Polyvinyl Acetate (PAN / VA)